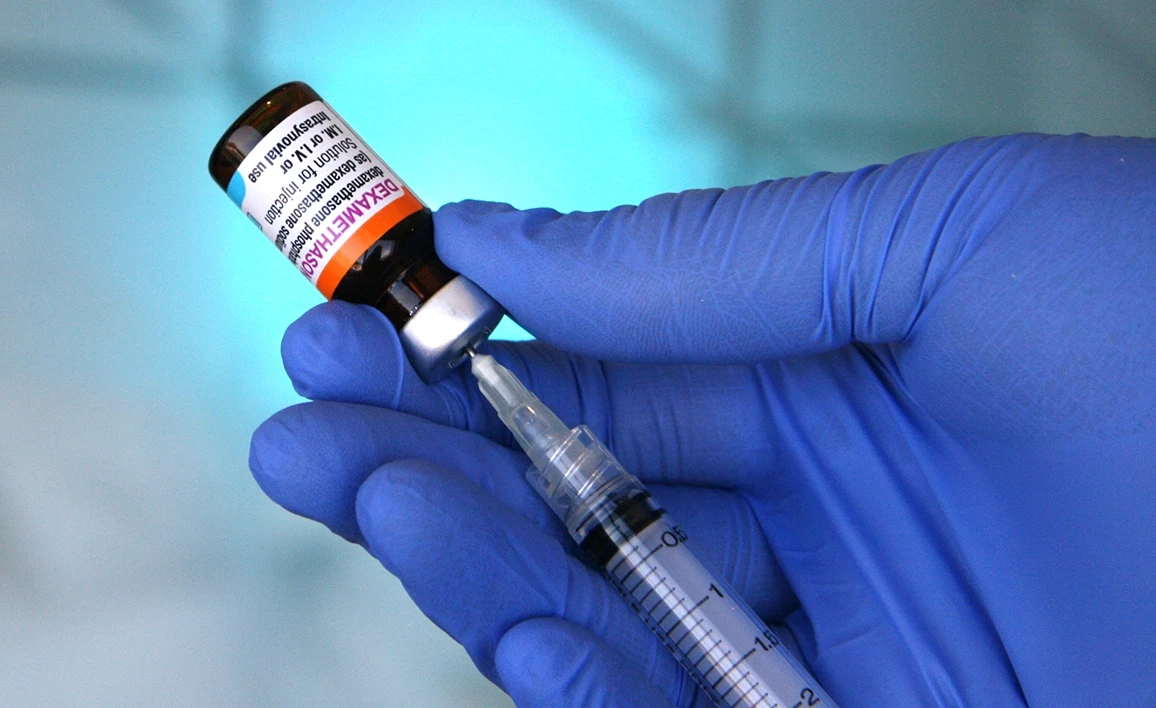
The Perioperative ADministration of Dexamethasone and Infection (PADDI) trial recruited 8880 patients from March 2016 to July 2019 from 55 sites across Australia, New Zealand, Hong Kong and South Africa. The manuscript detailing the trial results is now published in the New England Journal of Medicine.
We thank all our PADDI trial committee members, site investigators, fellows, trainees, research coordinators and the thousands of patients who were involved in the trial. Their efforts ultimately improve safety and care in the perioperative setting. The PADDI trial was the brainchild of Professor Tomás Corcoran and brings to fruition more than 10 years of concept development and preliminary research in the lead up to the major funding announcement in 2014, when it was awarded the highest valued National Health and Medical Research Council (NHMRC) project grant at that point of time in the history of the scheme.
Every year millions of patients undergo surgery worldwide. Surgical site infections (SSI) occur in up to 12 per cent of these patients. Complications resulting from SSI lead to increased morbidity and mortality, extended hospital stays, and carry an associated cost of up to US$10 billion per annum.
Dexamethasone is widely used by anaesthetists in the perioperative period, principally as an effective antiemetic to prevent postoperative nausea and vomiting (PONV). The molecular mechanisms underlying dexamethasone’s antiemetic action are not fully understood. However, because it is a potent glucocorticosteroid, it has immunosuppressive and hyperglycaemia effects. It is hypothesised that these actions may increase the risk of perioperative infections, particularly in patients with diabetes mellitus, who are already at increased risk of complications. Whether the use of dexamethasone in the perioperative period increases the risk of surgical site and other infections, has not been definitively established. This is an important health priority as in Australia alone up to one million patients will receive dexamethasone as part of their anaesthesia care annually.
This study aims to definitively address the impact of dexamethasone on surgical site infection and will be stratified according to diabetes status.
Study size
8880 adult patients
Study design
Multi-centre prospective, double blind, active control, parallel assessment, stratified, pragmatic non-inferiority safety and efficacy study.
Primary outcome
Surgical site infection within 30 days of surgery.
Secondary outcomes
Secondary outcomes include nausea and vomiting parameters, quality of perioperative analgesia, the incidence of chronic postsurgical pain, length of hospital stay, quality of recovery on days 1 and 30, inflammatory responses, glucose-related outcomes, infections at site other than the surgical site (pneumonia, bloodstream infection, urinary tract infection) up to 30 days, the incidence of sepsis and the incidence of wound dehiscence.
Study population
Adult patients, ASA physical status 1-4, undergoing elective or expedited (non-cardiac) surgery of at least two hours duration, using general anaesthesia with or without regional block, with a single (or multiple) surgical skin incision(s) of >5 cm in length, and a minimum anticipated hospital stay of at least one night.
The Australian National Health and Medical Research Council, Monash University and the Research Grant Council of Hong Kong.
Australian New Zealand Clinical Trials Registry registration number: ACTRN12614001226695
Professor Tomás Corcoran presented the results on 6 May 2021 with panelists Professor Paul Myles, A/Professor Trisha Peel, Professor Allen Cheng and Ms Karen Goulding. The recording of the webinar is now available on YouTube.
Professor Tomás Corcoran and Professor Paul Myles feature in a podcast explaining the PADDI trial and results. Listen here.
| Australian Hospitals |
| Alfred Hospital |
| Austin Hospital |
| Ballarat Health Services |
| Barwon Health |
| Blacktown Hospital |
| Box Hill Hospital |
| Cabrini Health |
| Canterbury Hospital |
| Concord Repatriation General Hospital |
| Dandenong Hospital |
| Epworth HealthCare Richmond |
| Fiona Stanley Hospital |
| Flinders Medical Centre |
| Gosford Hospital |
| Gold Coast University Hospital |
| John Hunter Hospital |
| King Edward Memorial Hospital |
| Mackay Base Hospital |
| Macquarie University Hospital |
| Maroondah Hospital |
| Monash Medical Centre |
| Peter MacCallum Cancer Centre |
| Prince of Wales Hospital NSW |
| Princess Alexandra Hospital |
| Redcliffe Hospital |
| Rockingham Hospital |
| Royal Adelaide Hospital |
| Royal Brisbane and Women’s Hospital |
| Royal Darwin Hospital |
| Royal Hobart Hospital |
| Royal Melbourne Hospital |
| Royal North Shore Hospital |
| Royal Perth Hospital |
| Royal Prince Alfred Hospital |
| Sir Charles Gairdner Hospital |
| St John of God Hospital Ballarat |
| St John of God Hospital Subiaco |
| St Vincent’s Hospital Melbourne |
| Sunshine Coast University Hospital |
| The Canberra Hospital |
| The Northern Hospital |
| The Tweed Hospital |
| Wagga Wagga Rural Referral Centre |
| Western Health |
| Westmead Hospital |
| Wollongong Hospital |
| New Zealand Hospitals |
| Auckland City Hospital |
| Auckland City Hospital CVICU |
| Christchurch Hospital |
| Hauora Tairawhiti Gisborne Hospital |
| Middlemore Hospital and Manukau Surgical Centre, Counties Manukau Health |
| South African Hospitals |
| Groote Schuur Hospital |